Dietary supplements are enormously popular. With an economic impact of $159 billion (2023), it is somewhat (but not much) smaller than Big Pharma at $800 billion (2022). The category includes thousands of products, generally consumed with the aim of health improvement or maintenance. Some supplements have defined ingredients that have been well studied, where the risk of toxicity is understood. Other supplements may be sourced from plants (e.g., herbs) and by definition will be multi-ingredient – after all, herbal remedies with medicinal effects are effectively unrefined drugs. Toxicity risks with herbal remedies may be less clear, owing to their different and varying constituents. Because there is generally less regulation of supplements versus prescription or over-the-counter drugs, there may also be discrepancies between what’s on the label compared to what’s actually in the bottle, increasing the uncertainty about what you’re actually buying when you buy a herbal remedy.
Liver harms from herbal remedies are increasing
The US-based Drug Induced Liver Injury Network (DILIN) collects and and analyzes cases of severe liver injury caused by prescription drugs, over-the-counter drugs, and alternative medicines/dietary supplements, including herbal remedies. It has previously reported that the proportion of drug-induced liver injury cases from dietary supplements nearly tripled from 7% in 2004-05 to 20% in 2013-14, with the most commonly implicated products including turmeric, kratom, green tea extract, and Garcinia cambogia. A similar trend was identified by the Acute Liver Failure Study Group, noting an increasing proportion of drug-induced liver injury attributed to dietary supplements, from 12.4% in 1998-2007 to 21.1% in 2007-15.
I have written about the National Health and Nutrition Examination Survey (NHANES) before which is a seemingly endless source of data on dietary supplement use. Surveys have been conducted every two years since 1999, and were only interrupted by the COVID-19 pandemic. In a recent study, entitled Estimated Exposure to 6 Potentially Hepatotoxic Botanicals in US Adults published in JAMA Network Open, the proportion of NHANES participants who reported consumption of six potentially hepatotoxic botanicals—turmeric or curcumin, green tea, Garcinia cambogia, black cohosh, red yeast rice, and ashwagandha—were identified. These were chosen because they are the most commonly implicated causes of supplement-induced liver injury based on the DILIN. Importantly while previous research has shown that supplements that can cause liver damage are frequently mislabeled, this study sought to better understand the frequency of use among Americans.
The NHANES survey collect a large amount of information, including detailed demographics, medical history, lifestyle factors, and dietary supplement, prescription and non-prescription drug use. This analysis looked at data collected between 2017 and March 2020. In this analysis, researchers looked for consumption of the six products listed above. Notably, doses were not assessed, nor was a direct confirmation of the ingredients in the supplements done. This was solely based on reporting of use, based on the brand name of the product reported.
In addition to the survey, about 95% of adults 18 years or older provided blood samples, which were tested with standard protocols. Participant’s livers were examined by Vibration-Controlled Transient Elastography which is a non-invasive method of evaluating the liver for damage.
Results
In a cohort of 9,685 adults, the mean age was 47.5 years, 52% female and 48% male. Racially, the participants included 16% Hispanic, 6% non-Hispanic Asian, 12% non-Hispanic Black, 62% non-Hispanic White, and 4% other races. Overall, 58% of adults reported using at least one herbal dietary supplement product in the past 30 days. (See below) Herbal dietary supplement users were generally older (mean age 51.9 vs 41.5 years), more likely to be female, non-Hispanic White, married, and had higher education levels compared to non-users. They were less likely to smoke, less likely to be below the poverty line, but had similar body mass index and alcohol use patterns compared to non-users. They were more likely to have conditions like hypertension, diabetes, coronary heart disease, stroke, arthritis, thyroid disorders, cancer, and liver issues compared to non-users. The median number of herbal dietary products used in 30 days was 1, with some using up to 22 products concurrently. Herbal dietary supplement users were also more likely to take prescription medications (70.1% vs 42.2%). They had had higher hemoglobin A1C and total cholesterol levels, consistent with their higher diabetes prevalence, but lower triglyceride levels. They also had lower serum alanine aminotransferase and alkaline phosphatase levels. Notably and importantly, no significant differences were observed between the groups for liver attenuation or stiffness scores.
Potentially Hepatotoxic Herbal Use
In this analysis, 8% of those surveyed used a botanical-containing dietary product in the past 30 days with 5% (350) used at least one of six potentially hepatotoxic botanicals, with turmeric/curcumin (236 users) and green tea (92 users) being the most common. Most botanical users (291) used only one of the six products, while a few (8) used three or more:
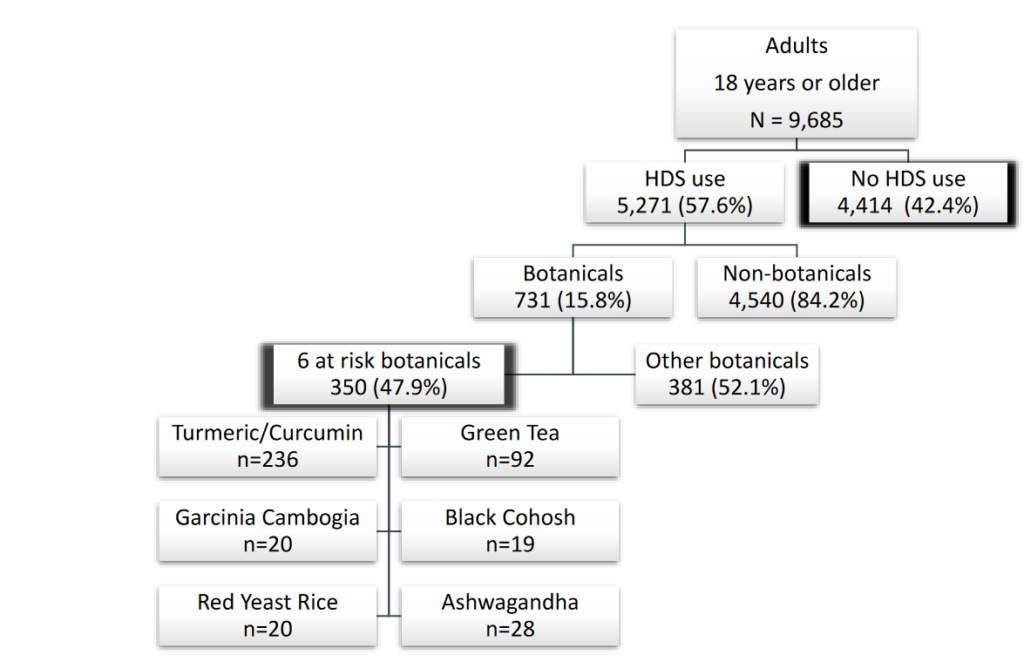
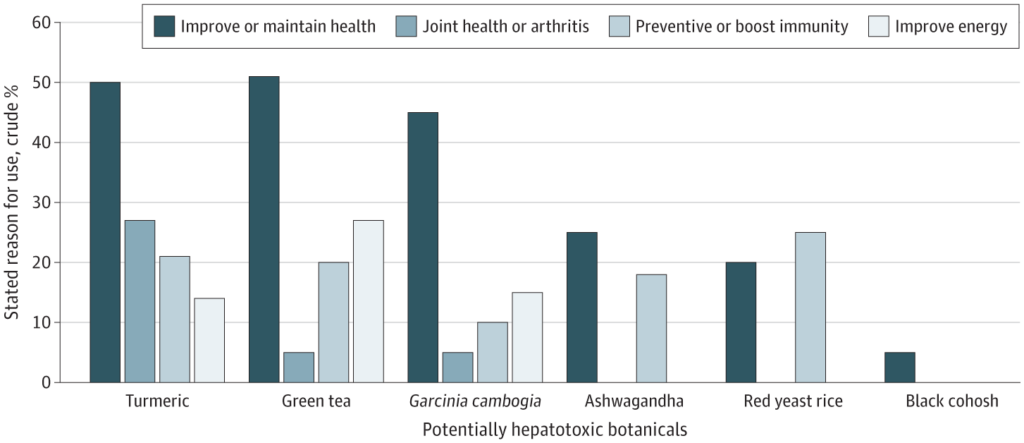
The majority of potentially hepatotoxic botanical users were self-medicating, with most not following healthcare provider recommendations. This included 88% for turmeric users, 80% for green tea, 63% for black cohosh, 40% for red yeast rice, 96% for ashwagandha and 100% for Garcinia cambogia users. Common reasons cited for using the products included improving or maintaining health, preventing health issues, or boosting immunity. Specific motivations included joint health or arthritis (27% of turmeric users), energy improvement (27% of green tea users), and weight loss (70% of Garcinia cambogia users). Black cohosh users (84%) mainly used it for hot flashes, and most users were women (88%). Red yeast rice was primarily used for heart health (90%) by older individuals with a high incidence of diabetes. (It is worth noting that there is little evidence to support these uses, and in some cases there are safer and more effective prescription drug alternatives available.)
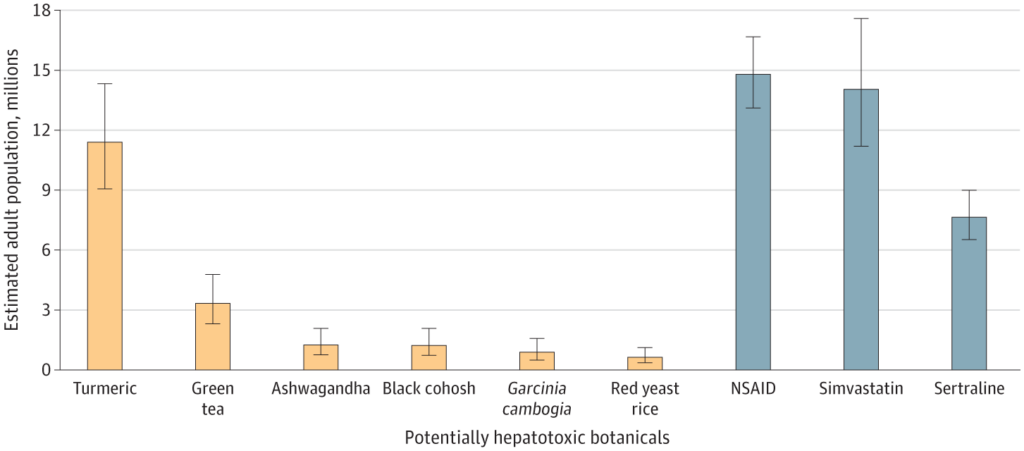
The prevalence of using these potentially hepatotoxic botanical products was compared to the use of known hepatotoxic prescription medications. About 14.8 million US adults used prescription NSAIDs, commonly for similar reasons as turmeric (pain or arthritis). Simvastatin, used for cardiovascular disease like red yeast rice, was used by around 14 million people. Additionally, 7.7 million individuals used sertraline, primarily prescribed for depression but also other mental health issues. Here is how usage of these herbal remedies compares, based on extrapolating the numbers from this survey. The numbers are non-trivial:
Widespread use of Potentially Hepatotoxic Herbal Remedies
Based on the results of this survey, it’s estimated that about 15 million US adults used at least one of these six potentially hepatotoxic herbal supplements in the past 30 days, a figure comparable to the number of people prescribed NSAIDs or simvastatin. Are there recorded harms from supplements? Yes: About 23,000 annual emergency visits and an increasing incidence of supplement-related liver injuries, which has led to severe outcomes such as liver transplants and deaths.
Just because a product is “natural” doesn’t mean it’s safe, especially when consumed regularly or in supplement format. For example, turmeric had few reports of association with liver damage until formulations were developed that were more easily absorbed – or combined with black pepper which also increases absorption. Reports of hepatic damage subsequently increased as this form of use became more popular.
Consumers are often hesitant (or may forget) to disclose their use of dietary supplements to health professionals, or they may not even view them as potential medicines that can have adverse effects. To support the identification and possible mitigation of liver injury from supplements, it’s essential that users understand that there are potential adverse effects with supplements, and, as well as potential negative interactions between herbs and medications. Given their frequency of use, unreported herbal remedy use should clearly be considered as a potential cause of otherwise-unexplained liver injury.

